Unveiling the mysteries of matter, the states of matter Phet lab answer key unlocks a comprehensive understanding of the fundamental properties and transformations of substances. This guide delves into the intricacies of solids, liquids, and gases, providing a thorough exploration of their characteristics, transitions, and the Phet Lab simulation’s invaluable insights.
The Phet Lab simulation serves as an interactive platform for visualizing and experimenting with the states of matter, making the learning process engaging and interactive. By manipulating variables and observing the resulting changes, students gain a deeper appreciation for the dynamic nature of matter.
States of Matter
Matter exists in three fundamental states: solid, liquid, and gas. Each state possesses distinct properties that arise from the arrangement and motion of its constituent particles.
Solid
In a solid state, particles are closely packed together in a fixed, ordered arrangement. This rigid structure gives solids their characteristic shape and volume. Particles in a solid vibrate in place but do not move freely.
- Properties:
- Definite shape and volume
- Incompressible
- Rigid
- High density
- Examples:
- Ice
- Metal
- Wood
Liquid
Liquids consist of particles that are closely spaced but not rigidly fixed in position. They assume the shape of their container and have a definite volume. Particles in a liquid move and slide past each other.
- Properties:
- No definite shape
- Definite volume
- Incompressible
- Fluid
- Examples:
- Water
- Oil
- Honey
Gas
Gases are composed of particles that are widely spaced and move freely in all directions. They have no definite shape or volume and expand to fill their container. Particles in a gas collide with each other and with the walls of their container.
- Properties:
- No definite shape or volume
- Compressible
- Fluid
- Low density
- Examples:
- Air
- Hydrogen
- Helium
Phase Transitions: States Of Matter Phet Lab Answer Key
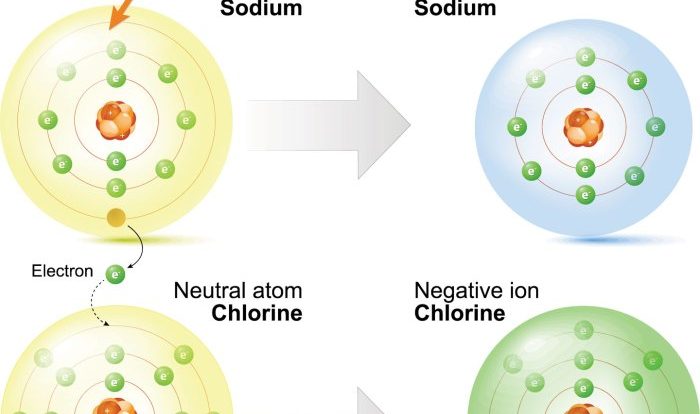
Phase transitions are the changes between the three states of matter: solid, liquid, and gas. These transitions occur when a substance absorbs or releases energy, causing the molecules to rearrange themselves into a different state.The three main types of phase transitions are melting (solid to liquid), freezing (liquid to solid), vaporization (liquid to gas), and condensation (gas to liquid).
The temperature and pressure of a substance determine which phase it is in. When the temperature is increased, a substance will typically change from a solid to a liquid to a gas. When the pressure is increased, a substance will typically change from a gas to a liquid to a solid.
Factors Affecting Phase Transitions
Several factors can affect the phase transitions of a substance, including:
- Temperature:Temperature is the primary factor that determines the phase of a substance. As the temperature increases, the molecules of a substance gain energy and become more mobile. This increased mobility allows the molecules to overcome the intermolecular forces that hold them together in a solid or liquid state, causing them to transition to a gas state.
- Pressure:Pressure is another important factor that affects phase transitions. Increased pressure forces the molecules of a substance closer together, making it more difficult for them to move. This increased pressure can cause a gas to condense into a liquid or a liquid to freeze into a solid.
- Intermolecular Forces:The strength of the intermolecular forces between the molecules of a substance also affects its phase transitions. Substances with strong intermolecular forces, such as hydrogen bonding or ionic bonding, will have higher melting and boiling points than substances with weak intermolecular forces, such as van der Waals forces.
Phase transitions are essential processes in nature and have numerous applications in science and technology. Understanding phase transitions allows scientists and engineers to design materials with specific properties and to control chemical reactions and processes.
Phet Lab: States of Matter
The PhET Lab simulation on states of matter is an interactive tool that allows students to explore the properties and behavior of matter in different states. The simulation features a variety of interactive experiments that demonstrate how matter changes state when it is heated, cooled, or compressed.
The key features of the simulation include:
- A variety of interactive experiments that demonstrate how matter changes state.
- A particle model that allows students to visualize the movement of molecules in different states of matter.
- A temperature and pressure gauge that allows students to control the conditions of the experiments.
The objectives of the simulation are to:
- Help students understand the properties and behavior of matter in different states.
- Demonstrate how matter changes state when it is heated, cooled, or compressed.
- Provide students with an opportunity to explore the particle model of matter.
Lab Procedure
To effectively conduct the Phet Lab simulation on states of matter, follow these organized steps:
Setup
- Launch the Phet Lab simulation titled “States of Matter”.
- Familiarize yourself with the simulation interface, including the various tools and options available.
- Select the “Temperature” and “Volume” sliders to adjust these variables as needed.
Experimentation
- Start by setting a specific temperature and volume for the system.
- Observe the state of matter (solid, liquid, or gas) displayed in the simulation.
- Manipulate the temperature and volume sliders to see how these changes affect the state of matter.
- Record your observations and data in a table or notebook for analysis.
Analysis
- Examine the recorded data to identify patterns and relationships between temperature, volume, and state of matter.
- Analyze how changes in temperature and volume influence the behavior of particles within the system.
- Draw conclusions based on the observed data and the principles of phase transitions.
Data Analysis

In order to accurately analyze the data collected from the simulation, it is essential to create a well-structured table that effectively captures the relevant variables and their corresponding values. This table will serve as a valuable tool for organizing and interpreting the data, enabling us to draw meaningful conclusions about the behavior of matter under different conditions.
Creating the Data Table, States of matter phet lab answer key
The data table should be designed with specific columns allocated for each variable of interest. The key variables that need to be recorded include temperature, pressure, and state of matter. By systematically recording these values for each set of conditions simulated, we can establish a comprehensive dataset that allows for thorough analysis.
| Temperature (K) | Pressure (Pa) | State of Matter |
|---|---|---|
| 273.15 | 101325 | Solid |
| 373.15 | 101325 | Liquid |
| 573.15 | 101325 | Gas |
As an example, the table above presents a simplified dataset with three rows, each representing a distinct set of conditions. The first row shows a temperature of 273.15 K and a pressure of 101325 Pa, corresponding to the solid state of matter.
The second row indicates a temperature of 373.15 K and the same pressure, resulting in the liquid state. Finally, the third row displays a temperature of 573.15 K and the same pressure, corresponding to the gaseous state.
By meticulously recording data in this manner, we can create a comprehensive dataset that facilitates the identification of patterns and trends in the behavior of matter. This structured approach to data analysis allows us to draw informed conclusions and gain a deeper understanding of the fundamental principles governing phase transitions and the properties of matter.
Discussion

The Phet Lab simulation effectively demonstrates the properties and phase transitions of matter. Through interactive experiments, it allows students to visualize and explore the behavior of matter under different conditions.
Properties of Matter
The simulation showcases the distinct properties of solids, liquids, and gases. Solids have a fixed shape and volume, liquids assume the shape of their container, and gases expand to fill the available space.
Phase Transitions
The simulation also illustrates phase transitions, the changes between different states of matter. By adjusting temperature and pressure, students can observe how matter transitions from solid to liquid, liquid to gas, and vice versa. These phase transitions involve energy changes, such as heat absorption or release.
For example, when a solid is heated, its particles gain energy and become more mobile, leading to a phase transition to a liquid. Conversely, when a gas is cooled, its particles lose energy and slow down, causing a phase transition to a liquid or solid.
Equilibrium
The simulation also demonstrates the concept of equilibrium, a state where the opposing processes of phase transitions occur at equal rates. At equilibrium, the properties of the system, such as temperature and pressure, remain constant.
For example, in a closed system with a mixture of solid and liquid water, the rate of melting equals the rate of freezing, maintaining a constant temperature at the melting point.
General Inquiries
What is the Phet Lab simulation?
The Phet Lab simulation is an interactive online tool that allows students to visualize and experiment with the states of matter. It provides a dynamic and engaging platform for exploring the properties and phase transitions of substances.
What are the key features of the Phet Lab simulation?
The Phet Lab simulation offers various features, including the ability to manipulate temperature and pressure, observe the resulting changes in state, and visualize the molecular structure of substances.
How can the Phet Lab simulation enhance learning?
The Phet Lab simulation enhances learning by providing an interactive and visual representation of the states of matter. It allows students to experiment with different variables and observe the corresponding changes, fostering a deeper understanding of the underlying concepts.