Embark on an educational journey with our comprehensive oxidation numbers worksheet and answers, meticulously crafted to provide a profound understanding of this fundamental chemical concept. This guide unravels the intricacies of oxidation numbers, empowering you with the knowledge and skills to navigate redox reactions and delve into advanced chemical theories.
Our interactive worksheet poses thought-provoking problems, guiding you through the intricacies of assigning oxidation numbers to various elements and compounds. Accompanying answer key elucidates each step, ensuring a thorough comprehension of the underlying principles. By engaging with these exercises, you will master the art of oxidation number assignment, laying a solid foundation for further exploration in chemistry.
Oxidation Numbers: Oxidation Numbers Worksheet And Answers

Oxidation numbers, also known as oxidation states, are numerical values assigned to atoms in a molecule or ion to represent the degree of oxidation or reduction of that atom.
An atom’s oxidation number reflects the number of electrons it has lost or gained relative to its neutral state. Positive oxidation numbers indicate a loss of electrons, while negative oxidation numbers indicate a gain of electrons.
Rules for Assigning Oxidation Numbers
- The oxidation number of an element in its elemental form is 0.
- The oxidation number of a monatomic ion is equal to its charge.
- The sum of the oxidation numbers of all atoms in a molecule or ion must be equal to the charge of the molecule or ion.
- In a covalent bond, the more electronegative atom has a negative oxidation number, while the less electronegative atom has a positive oxidation number.
- In polyatomic ions, the oxidation number of the central atom is usually determined by the charge of the ion and the oxidation numbers of the surrounding atoms.
Examples of Oxidation States, Oxidation numbers worksheet and answers
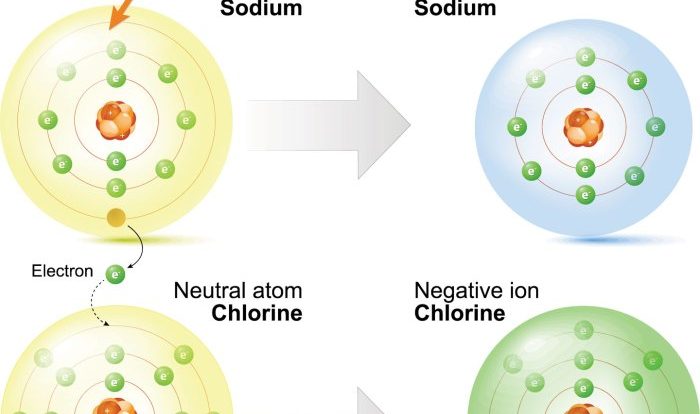
- Sodium (Na) in NaCl has an oxidation number of +1.
- Chlorine (Cl) in NaCl has an oxidation number of -1.
- Oxygen (O) in H2O has an oxidation number of -2.
- Hydrogen (H) in H2O has an oxidation number of +1.
- Iron (Fe) in Fe2O3 has an oxidation number of +3.
FAQ Summary
What is the significance of oxidation numbers in chemistry?
Oxidation numbers provide a systematic approach to understanding the electron transfer processes that occur in chemical reactions, enabling the prediction and balancing of redox reactions.
How can I determine the oxidation number of an element in a compound?
Follow the established rules for assigning oxidation numbers, considering the element’s position in the periodic table, its electronegativity, and the overall charge of the compound.
What are some common mistakes to avoid when assigning oxidation numbers?
Be cautious of assigning incorrect oxidation numbers to elements based on their group number or assuming that all bonds are purely ionic or covalent.