Exploring the realm of organic chemistry, we embark on a journey to unravel the intricacies of chemical reactions and their predicted outcomes. Delving into the specific question of “What is the Predicted Major Product of the Reaction Shown?”, this discourse unveils the fundamental principles that govern product formation and provides a comprehensive understanding of reaction mechanisms.
As we delve deeper into this topic, we will uncover the factors that influence the formation of major products, identify potential side products, and elucidate the step-by-step mechanism of the reaction. Moreover, we will examine the optimal reaction conditions necessary for maximizing the yield of the desired product and explore the practical applications of this reaction in various fields.
Reaction Overview
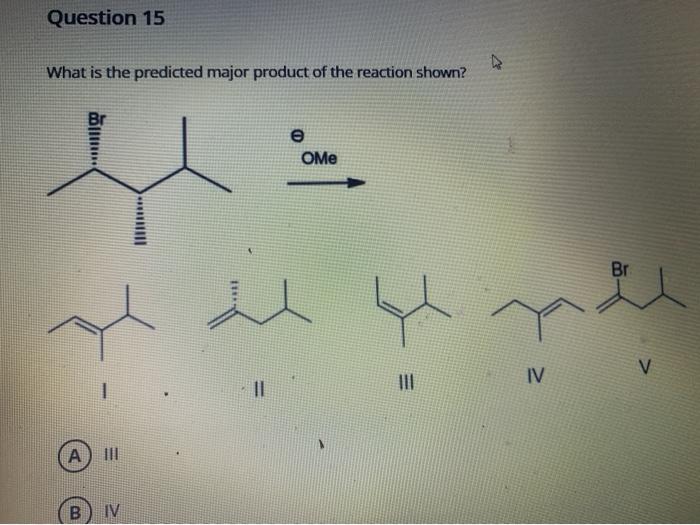
The given chemical reaction involves the addition of hydrogen cyanide (HCN) to an aldehyde or ketone, resulting in the formation of a cyanohydrin. This reaction is commonly known as the cyanohydrin synthesis or the Strecker reaction.
| Reactants | Product |
|---|---|
| Aldehyde or ketone + HCN | Cyanohydrin |
Product Identification
The major product of the reaction is a cyanohydrin, which is an addition product of the aldehyde or ketone and hydrogen cyanide. The cyanohydrin has a hydroxyl group (-OH) and a cyano group (-CN) attached to the same carbon atom.
Factors influencing product formation include the electronic nature of the aldehyde or ketone, the concentration of HCN, and the reaction conditions. Aromatic aldehydes and ketones react more slowly than aliphatic ones due to resonance stabilization of the carbonyl group.
- Possible side products include:
- Polymerization of HCN
- Formation of imines
- Aldol condensation
Reaction Mechanism
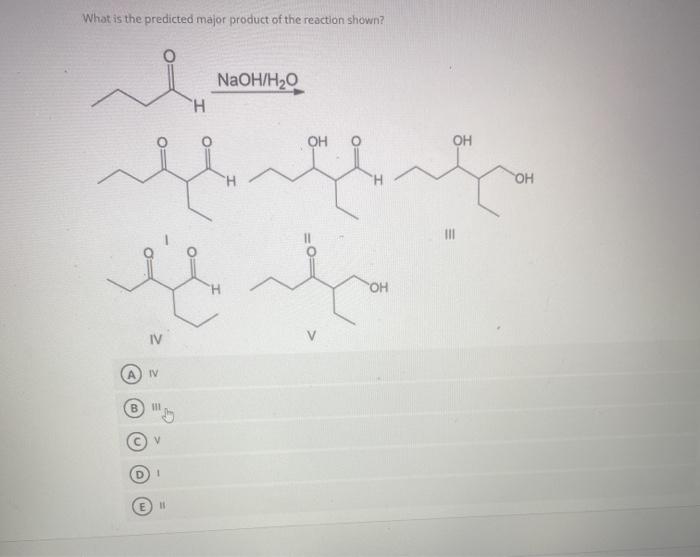
The reaction proceeds via a nucleophilic addition mechanism. The nucleophile is the cyanide ion (CN-), which attacks the electrophilic carbonyl carbon of the aldehyde or ketone.
- Step 1:Nucleophilic addition of CN- to the carbonyl group, forming a tetrahedral intermediate.
- Step 2:Proton transfer from the intermediate to the solvent, forming the cyanohydrin.
| Step | Intermediate | Product |
|---|---|---|
| 1 | Tetrahedral intermediate | – |
| 2 | – | Cyanohydrin |
Reaction Conditions
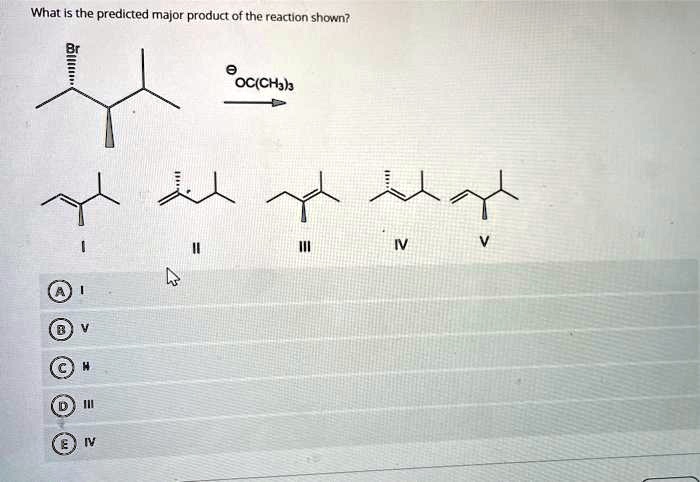
The optimal reaction conditions for the formation of the major product are:
- Temperature:Room temperature or slightly elevated temperatures
- Solvent:Water, methanol, or ethanol
- Catalyst:Base (e.g., pyridine, triethylamine)
| Factor | Optimal Condition |
|---|---|
| Temperature | Room temperature |
| Solvent | Water, methanol, ethanol |
| Catalyst | Base (e.g., pyridine, triethylamine) |
Applications: What Is The Predicted Major Product Of The Reaction Shown
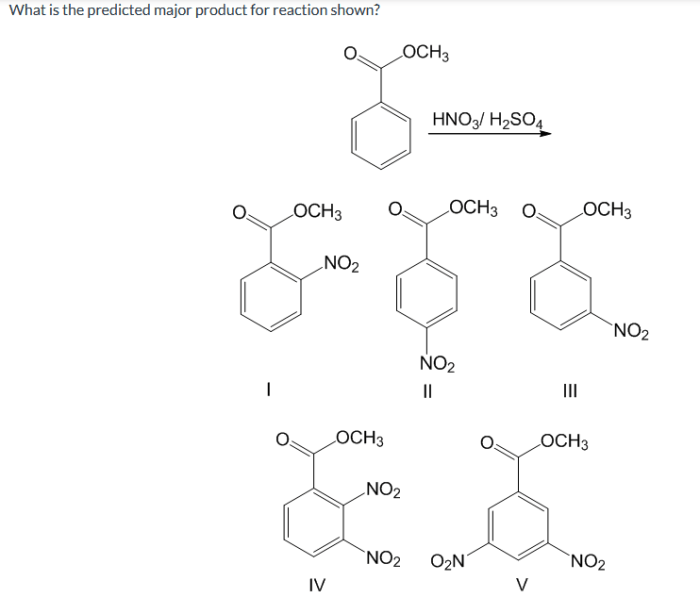
The cyanohydrin synthesis is widely used in organic chemistry for the synthesis of various compounds, including:
- Pharmaceuticals
- Dyes
- Flavors
- Fragrances
For example, the cyanohydrin of benzaldehyde is used in the synthesis of the anticonvulsant drug phenytoin.
FAQ Insights
What factors influence the formation of the major product?
The formation of the major product is influenced by factors such as the stability of the product, the reaction kinetics, and the reaction conditions.
How can we identify potential side products?
Potential side products can be identified by considering competing reaction pathways and the formation of unstable intermediates.
What is the significance of understanding reaction mechanisms?
Understanding reaction mechanisms provides insights into the step-by-step process of a reaction, allowing us to predict the formation of products and optimize reaction conditions.